Food, Beverage, and Feedstock Processing Facility Wastewater: a Unique and Underappreciated Source of Contaminants to U.S. Streams | Environmental Science & Technology
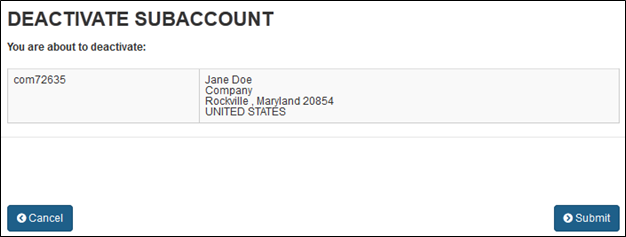
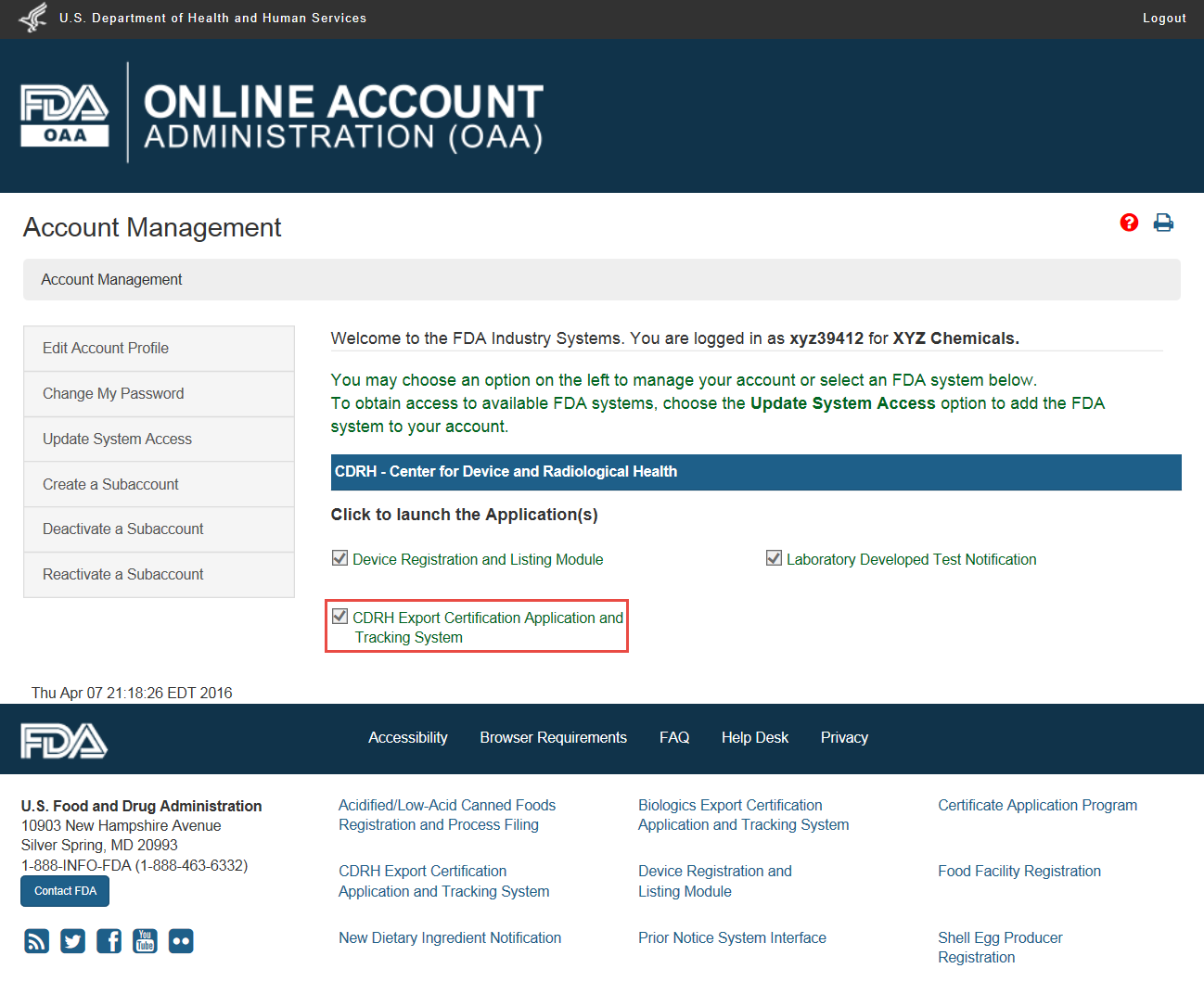
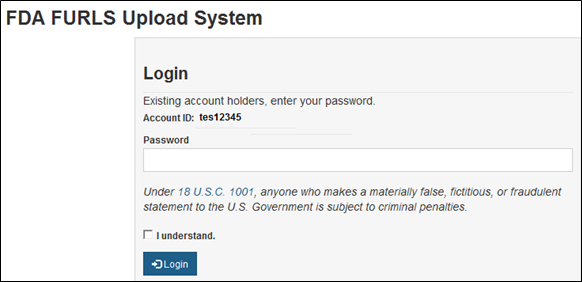
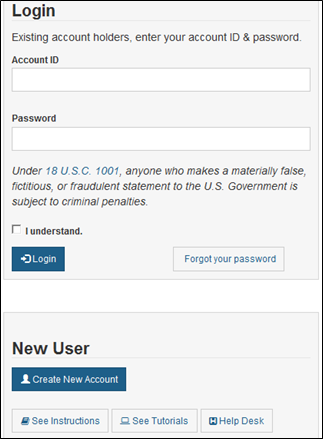
Page 1 of 2 Food Facility Registration > FDA Industry Systems User Guide: Account Management 1/14/2014 http://www.fda.gov/Foo
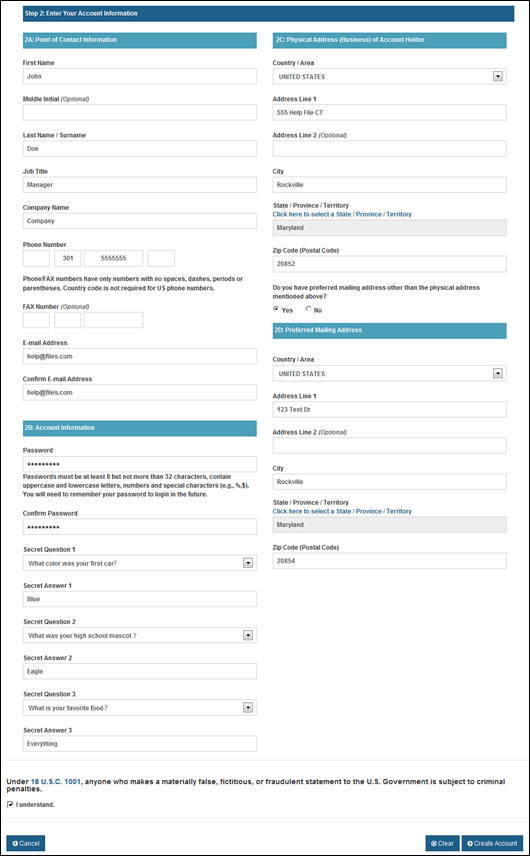
Device Registration and Listing Module Public reporting burden for this collection of information on form FDA 3673 is estimated